Spina bifida
Introduction
The human nervous system develops from a small, specialised plate of cells along the back of an embryo (called the neural plate). Early in development, the edges of this plate begin to curl up toward each other, creating the neural tube—a narrow sheath that closes to form the brain and spinal cord of the embryo. As development progresses, the top of the tube becomes the brain and the remainder becomes the spinal cord. This process is usually complete by the 28th day of pregnancy. But if problems occur during this process, the result can be brain disorders called neural tube defects, including spina bifida.
What is spina bifida?
Spina bifida, which literally means “split spine,” is characterized by the incomplete development of the brain, spinal cord, and/or meninges (the protective covering around the brain and spinal cord). It is the most common neural tube defect
What are the different types of spina bifida?
There are four types of spina bifida: occulta, closed neural tube defects, meningocele, and myelomeningocele.
Occulta is the mildest and most common form in which one or more vertebrae are malformed. The name “occulta,” which means “hidden,” indicates that a layer of skin covers the malformation, or opening in the vertebrae. This form of spina bifida, present in 10-20 percent of the general population, rarely causes disability or symptoms.
Closed neural tube defects make up the second type of spina bifida. This form consists of a diverse group of defects in which the spinal cord is marked by malformations of fat, bone, or meninges. In most instances there are few or no symptoms; in others the malformation causes incomplete paralysis with urinary and bowel dysfunction.
In the third type, meningocele, spinal fluid and meninges protrude through an abnormal vertebral opening; the malformation contains no neural elements and may or may not be covered by a layer of skin. Individuals with meningocele usually have no neurological symptoms
Myelomeningocele, the fourth form, is the most severe and occurs when the spinal cord/neural elements are exposed through the opening in the spine, resulting in partial or complete paralysis of the parts of the body below the spinal opening. The impairment may be so severe that the affected individual is unable to walk and may have bladder and bowel dysfunction.
What causes spina bifida?
The exact cause of spina bifida remains a mystery. No one knows what disrupts complete closure of the neural tube, causing this malformation to develop. Scientists suspect the factors that cause spina bifida are multiple: genetic, nutritional, and environmental factors all play a role. Research studies indicate that insufficient intake of folic acid—a common B vitamin—in the mother’s diet is a key factor in causing spina bifida and other neural tube defects. Prenatal vitamins typically contain folic acid as well as other vitamins. (See “Can the disorder be prevented?” for more information on folic acid.)
What are the signs and symptoms of spina bifida?
The symptoms of spina bifida vary from person to person, depending on the type and level of involvement. Closed neural tube defects are often recognized early in life due to an abnormal tuft or clump of hair or a small dimple or birthmark on the skin at the site of the spinal malformation.
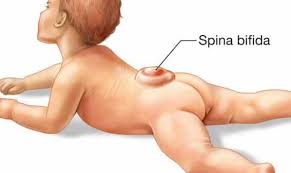
Meningocele and myelomeningocele generally involve a fluid-filled sac—visible on the back—protruding from the spinal canal. In meningocele, the sac may be covered by a thin layer of skin. In most cases of myelomeningocele, there is no layer of skin covering the sac and an area of abnormally developed spinal cord tissue is usually exposed.
What are the complications of spina bifida?
Complications of spina bifida can range from minor physical problems with little functional impairment to severe physical and mental disabilities. It is important to note, however, that most people with spina bifida are of normal intelligence. Spina bifida’s impact is determined by the size and location of the malformation, whether it covered, and which spinal nerves are involved. All nerves located below the malformation are affected to some degree. Therefore, the higher the malformation occurs on the back, the greater the amount of nerve damage and loss of muscle function and sensation.
In addition to abnormal sensation and paralysis, another neurological complication associated with spina bifida is Chiari II malformation—a condition common in children with myelomeningocele—in which the brain stem and the cerebellum (hindbrain) protrude downward into the spinal canal or neck area. This condition can lead to compression of the spinal cord and cause a variety of symptoms including difficulties with feeding, swallowing, and breathing control; choking; and changes in upper arm function (stiffness, weakness).
Chiari II malformation may also result in a blockage of cerebrospinal fluid, causing a condition called hydrocephalus, which is an abnormal buildup of cerebrospinal fluid in and around the brain. Cerebrospinal fluid is a clear liquid that surrounds the brain and spinal cord. The buildup of fluid puts damaging pressure on these structures. Hydrocephalus is commonly treated by surgically implanting a shunt—a hollow tube—in the brain to drain the excess fluid into the abdomen.
Some newborns with myelomeningocele may develop meningitis, an infection in the meninges. Meningitis may cause brain injury and can be life-threatening.
Children with both myelomeningocele and hydrocephalus may have learning disabilities, including difficulty paying attention, problems with language and reading comprehension, and trouble learning math.
Additional problems such as latex allergies, skin problems, gastrointestinal conditions, and depression may occur as children with spina bifida get older.
How is it diagnosed?
In most cases, spina bifida is diagnosed prenatally, or before birth. However, some mild cases may go unnoticed until after birth (postnatal). Very mild forms (spinal bifida occulta), in which there are no symptoms, may never be detected.
Prenatal Diagnosis
The most common screening methods used to look for spina bifida during pregnancy are second trimester (16-18 weeks of gestation) maternal serum alpha fetoprotein (MSAFP) screening and foetal ultrasound. The MSAFP screen measures the level of a protein called alpha-fetoprotein (AFP), which is made naturally by the foetus and placenta. During pregnancy, a small amount of AFP normally crosses the placenta and enters the mother’s bloodstream. If abnormally high levels of this protein appear in the mother’s bloodstream, it may indicate that the foetus has an “open” (not skin-covered) neural tube defect. The MSAFP test, however, is not specific for spina bifida and requires correct gestational dates to be most accurate; it cannot definitively determine that there is a problem with the foetus. If a high level of AFP is detected, the doctor may request additional testing, such as an ultrasound or amniocentesis to help determine the cause.
The second trimester MSAFP screen described above may be performed alone or as part of a larger, multiple-marker screen. Multiple-marker screens look not only for neural tube defects, but also for other birth defects, including Down syndrome and other chromosomal abnormalities. First trimester screens for chromosomal abnormalities also exist but signs of spina bifida are not evident until the second trimester when the MSAFP screening is performed.
Amniocentesis—an exam in which the doctor removes samples of fluid from the amniotic sac that surrounds the foetus—may also be used to diagnose spina bifida. Although amniocentesis cannot reveal the severity of spina bifida, finding high levels of AFP and other proteins may indicate that the disorder is present.
Postnatal Diagnosis
Mild cases of spina bifida (occulta, closed) not diagnosed during prenatal testing may be detected postnatally by plain film X-ray examination. Individuals with the more severe forms of spina bifida often have muscle weakness in their feet, hips, and legs that result in deformities that may be present at birth. Doctors may use magnetic resonance imaging (MRI) or a computed tomography (CT) scan to get a clearer view of the spinal cord and vertebrae. If hydrocephalus is suspected, the doctor may request a CT scan and/or X-ray of the skull to look for extra cerebrospinal fluid inside the brain.
How is spina bifida treated?
There is no cure for spina bifida. The nerve tissue that is damaged cannot be repaired, nor can function be restored to the damaged nerves. Treatment depends on the type and severity of the disorder. Generally, children with the mildest form need no treatment, although some may require surgery as they grow. Those with open defects need surgery in the first 48hrs of life to close the defect.
The key early priorities for treating myelomeningocele are to prevent infection from developing in the exposed nerves and tissue through the spinal defect, and to protect the exposed nerves and structures from additional trauma. Typically, a child born with spina bifida will have surgery to close the defect and minimize the risk of infection or further trauma within the first few days of life.
Selected medical centres perform foetal surgery for treatment of myelomeningocele through a National Institutes of Health experimental protocol (Management of Myelomeningocele Study, or MOMS). Foetal surgery is performed in utero (within the uterus) and involves opening the mother’s abdomen and uterus and sewing shut the abnormal opening over the developing baby’s spinal cord. Some doctors believe the earlier the defect is corrected, the better the baby’s outcome. Although the procedure cannot restore lost neurological function, it may prevent additional loss from occurring.
The surgery is considered experimental and there are risks to the foetus as well as to the mother. The major risks to the foetus are those that might occur if the surgery stimulates premature delivery, such as organ immaturity, brain haemorrhage, and death. Risks to the mother include infection, blood loss leading to the need for transfusion, gestational diabetes, and weight gain due to bed rest. This treatment however is not available in Zimbabwe and most medical centres throughout the world. Even in developed countries.
Twenty to 50 percent of children with myelomeningocele develop a condition called progressive tethering, or tethered cord syndrome; their spinal cord become fastened to an immovable structure—such as overlying membranes and vertebrae—causing the spinal cord to become abnormally stretched with the child’s growth. This condition can cause loss of muscle function to the legs, as well as changes in bowel and bladder function. Early surgery on a tethered spinal cord may allow the child to return to their baseline level of functioning and prevent further neurological deterioration.
Some children will need subsequent surgeries to manage problems with the feet, hips, or spine. Individuals with hydrocephalus generally will require additional surgeries to replace the shunt, which can be outgrown or become clogged or infected.
Some individuals with spina bifida require assistive devices such as braces, crutches, or wheelchairs. The location of the malformation on the spine often indicates the type of assistive devices needed. Children with a defect high on the spine will have more extensive paralysis and will often require a wheelchair, while those with a defect lower on the spine may be able to use crutches, leg braces, or walkers. Beginning special exercises for the legs and feet at an early age may help prepare the child for walking with those braces or crutches when he or she is older.
Treatment for bladder and bowel problems typically begins soon after birth, and may include bladder catheterizations and bowel management regimens.
Can the disorder be prevented?
Folic acid, also called folate, is an important vitamin in the development of a healthy foetus. Although taking this vitamin cannot guarantee having a healthy baby, it can help. Recent studies have shown that by adding folic acid to their diets, women of childbearing age significantly reduce the risk of having a child with a neural tube defect, such as spina bifida. Therefore, it is recommended that all women of childbearing age consume 400 micrograms of folic acid daily. Foods high in folic acid include dark green vegetables, egg yolks, and some fruits. Many foods—such as some breakfast cereals, enriched breads, flours, pastas, rice, and other grain products—are now fortified with folic acid. Many multivitamins contain the recommended dosage of folic acid as well.
Women who already have a child with spina bifida, who have spina bifida themselves, or who have already had a pregnancy affected by any neural tube defect are at greater risk of having another child with spina bifida or another neural tube defect; 5-10 times the risk to the general population. These women may benefit from taking a higher daily dose of folic acid before they consider becoming pregnant.
What is the prognosis?
Children with spina bifida can lead active lives. Prognosis, activity, and participation depend on the number and severity of abnormalities and associated personal and environmental factors. Most children with the disorder have normal intelligence and can walk, often with assistive devices. If learning problems develop, appropriate educational interventions are helpful.
“Spina Bifida Fact Sheet”, NINDS, Publication date June 2013NIH Publication No. 13-309

